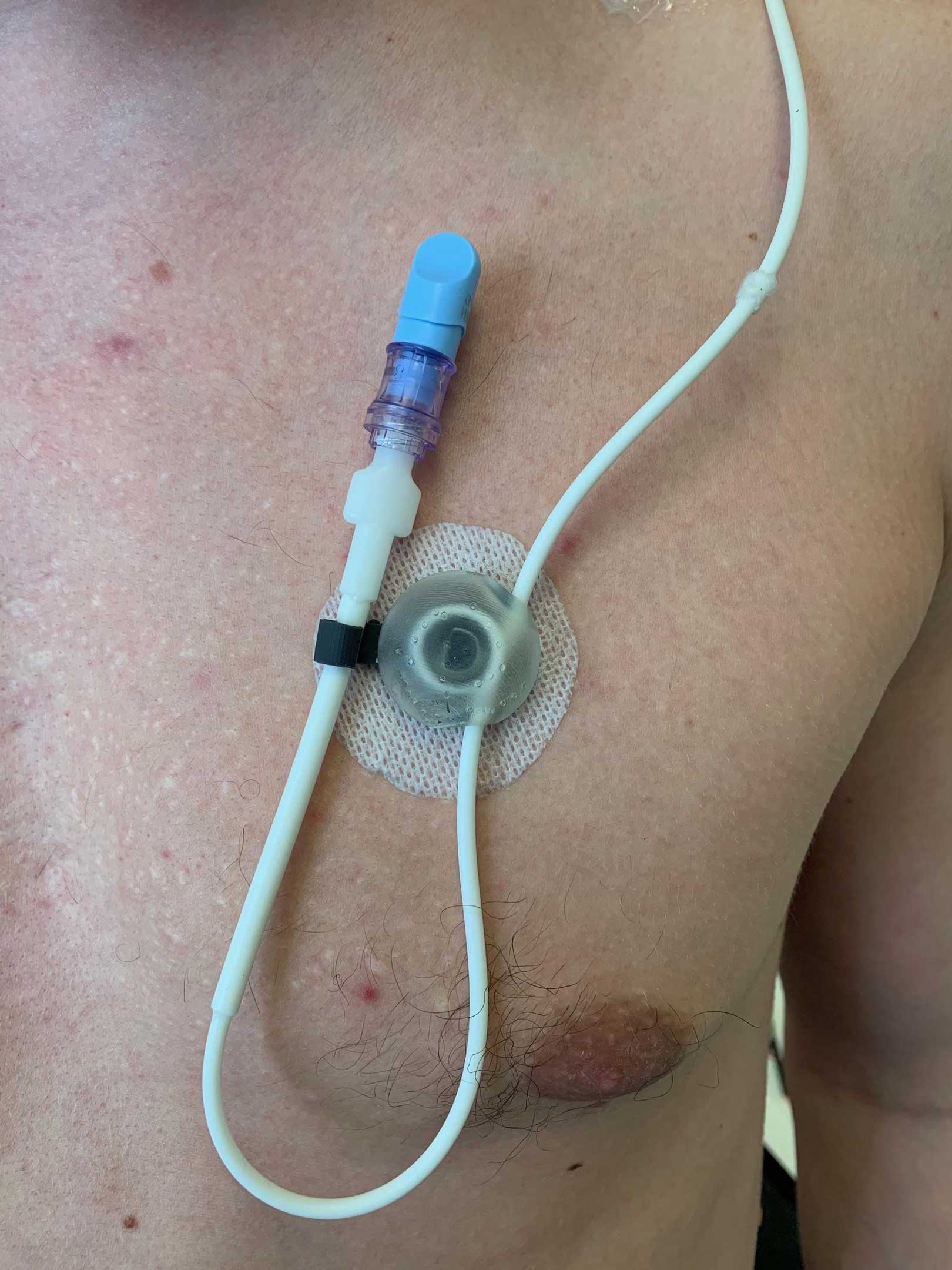
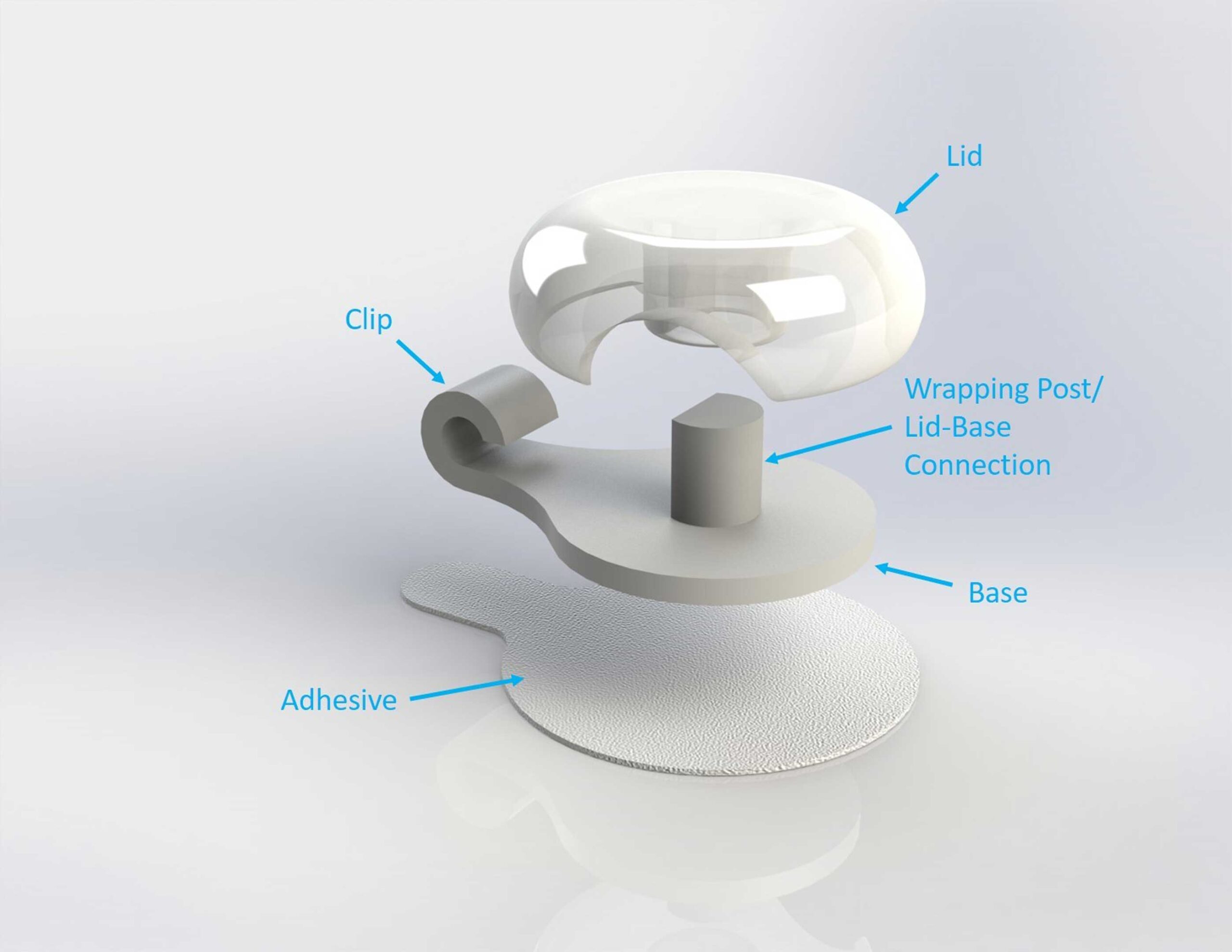
CVAD Securement
Product Description
This product is designed to provide securement of a Central Venous Access Device (CVAD), in order to reduce complications, such as line damage, inaccessability, discomfort, or dislodgement.
Objective
In a team of 4, design a device for CVAD securement (patent pending), working with a client (Surgeon at the Children’s Hospital Colorado).
Limitations
$10,000 budget
Duration
One academic year (Fall 2019 – Spring 2020)

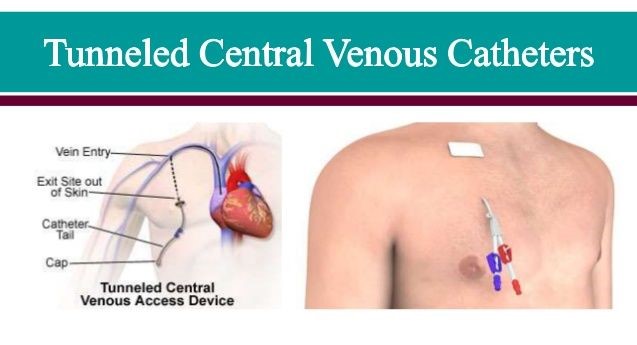
Background Info / Research
External tunneled central venous access devices (CVAD) play an essential role in the management of many patients with acute and chronic illnesses, however, current dressing and securement methods for these devices do not provide the comfort and protection that patients need. Current methods do not adequately protect patients from bloodstream infections, nor do they safeguard from inadvertent dislodgement, which may require removal and/or replacement of the CVAD. Current external tunneled CVAD dressing and securement components can also be uncomfortable, especially for younger patients with smaller torsos and more sensitive skin. The external tunneled CVAD market is expected to register a 5.5% compound annual growth rate over the next five years.
Initial Requirements (from client)
Secure catheter tubing and ports.
Encompass both catheter and exit site (along with Biopatch used at exit site).
Include a base component and a hinged lid, and be sterilizable.
Device must be “as small as possible” (capable of fitting patients from 31 days to 18 years of age).
Existing Products
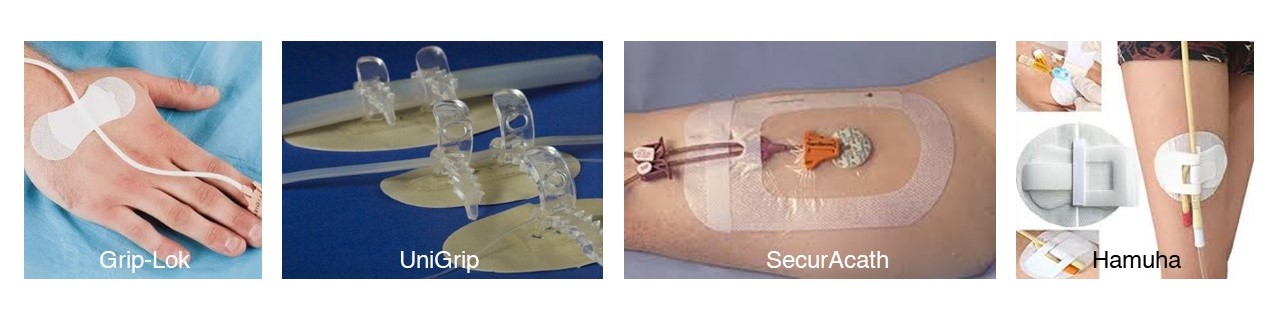
Initial Nurse (User) Interview
Long-term pressure points can lead to skin damage and/or breakdown.
Must be able to see the exit site in order to check for infection.
Device must be small/discrete.
Usage must be simple and intuitive.
Prototype Rounds 1 & 2

User Feedback
Clear prototype is favorite due to small foot print and profile, as well as having a clear lid.
Changing anything that has to do with the exit site (e.g. creating a custom occlusive dressing or eliminating the occlusive dressing over the exit site) is not advised. The current occlusive dressing protects against infection, and getting people to agree with any change will be difficult.
There should be a clip for the end of the catheter, but with enough slack to handle the port for catheter usage.
Some patients may not want the device to be directly over the exit site. They may want the freedom to move it around a bit.
Testing
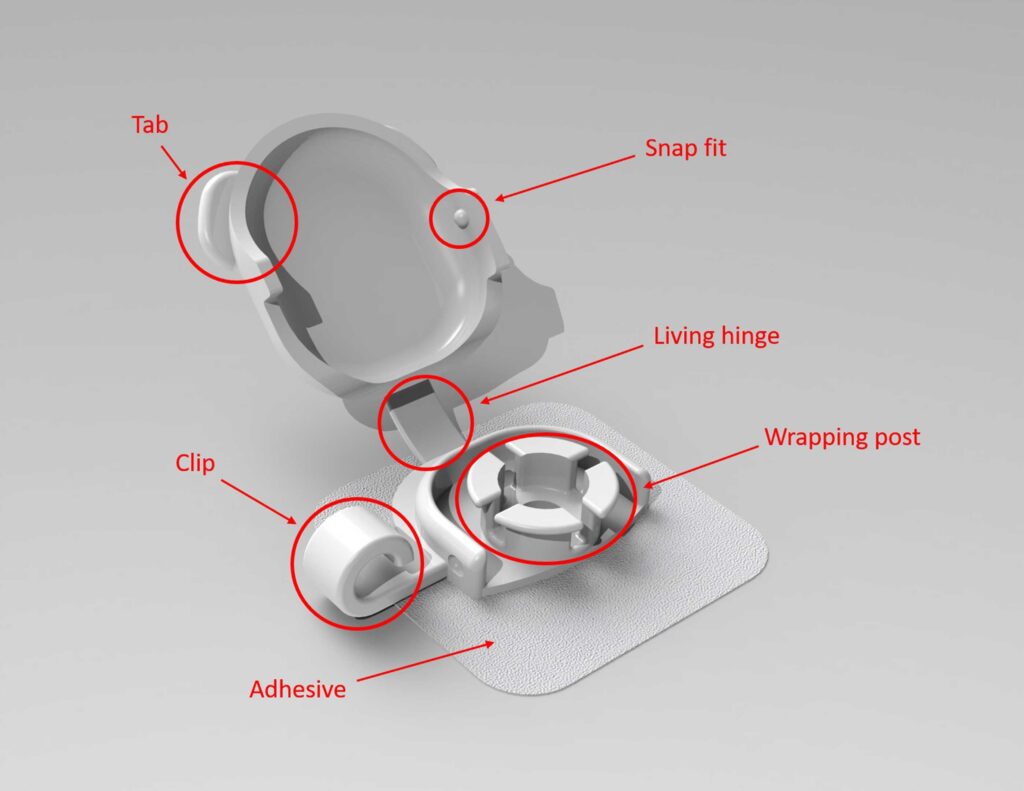
Several types of testing were involved while designing this device. Because these catheters administer life-saving fluids, a testing rig had to be 3D printed and used to make sure that the wrapping post within the device had a large enough radius to ensure that fluid flowing through the catheter would not be inhibited, regardless of the catheter size. Many iterations of the living hinge design were created as well, using materials and thicknesses based on extensive research. We conducted failure tests (counting the number of “bends” until breaking) until a design/material was successful. A living hinge was necessary to lower production cost and to ensure that the lid did not become a choking hazzard for small children.

Initial "Final" Design
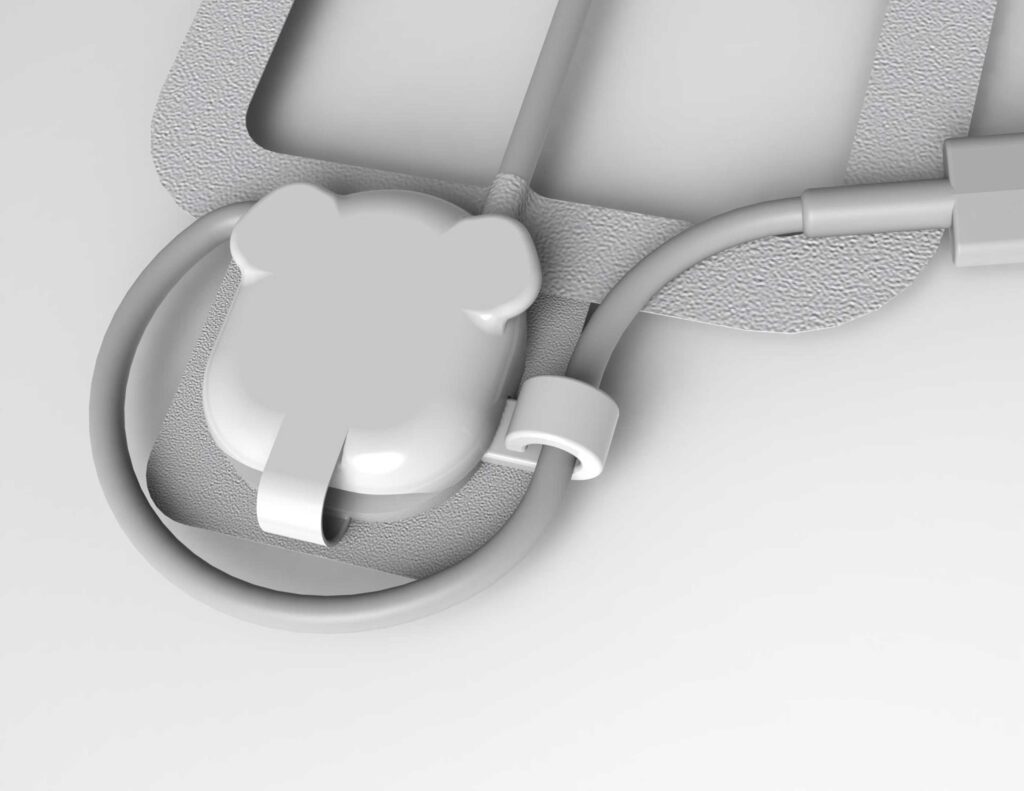
An Unexpected turn

After we developed the design shown above, the next step in the process was filing for a provisional patent and preparing for clinical trials. Our client was happy with the design, but now mentioned that there needed to be a “100% guarantee that the catheter would not be pinched”. For this to be the case, our design could not include a hinged lid. Here we used a bit of biomimicry: drawing insipiration from the jellyfish, we took pieces of our initial design that we knew worked well, and designed a device that utilized a soft, flexible lid that allowed the catheter to be slid underneath, and that would not pinch the catheter, even if user error somehow occured.
Design Iterations / Material Selection
Final Requirements
Compatible
An overall small size allows the device to be compatible for patients ranging from 31 days to adulthood.

Clear
A clear, flexible lid allows for viewing of the catheter while the device is in use in order to ensure that there are no pinches in the line.

Intuitive
A D-shaped post allows for ease of assembly, while a simple overall design allows for easy usage, with very few steps and directions needed.

Final Product